- Your cart is empty
- Continue Shopping
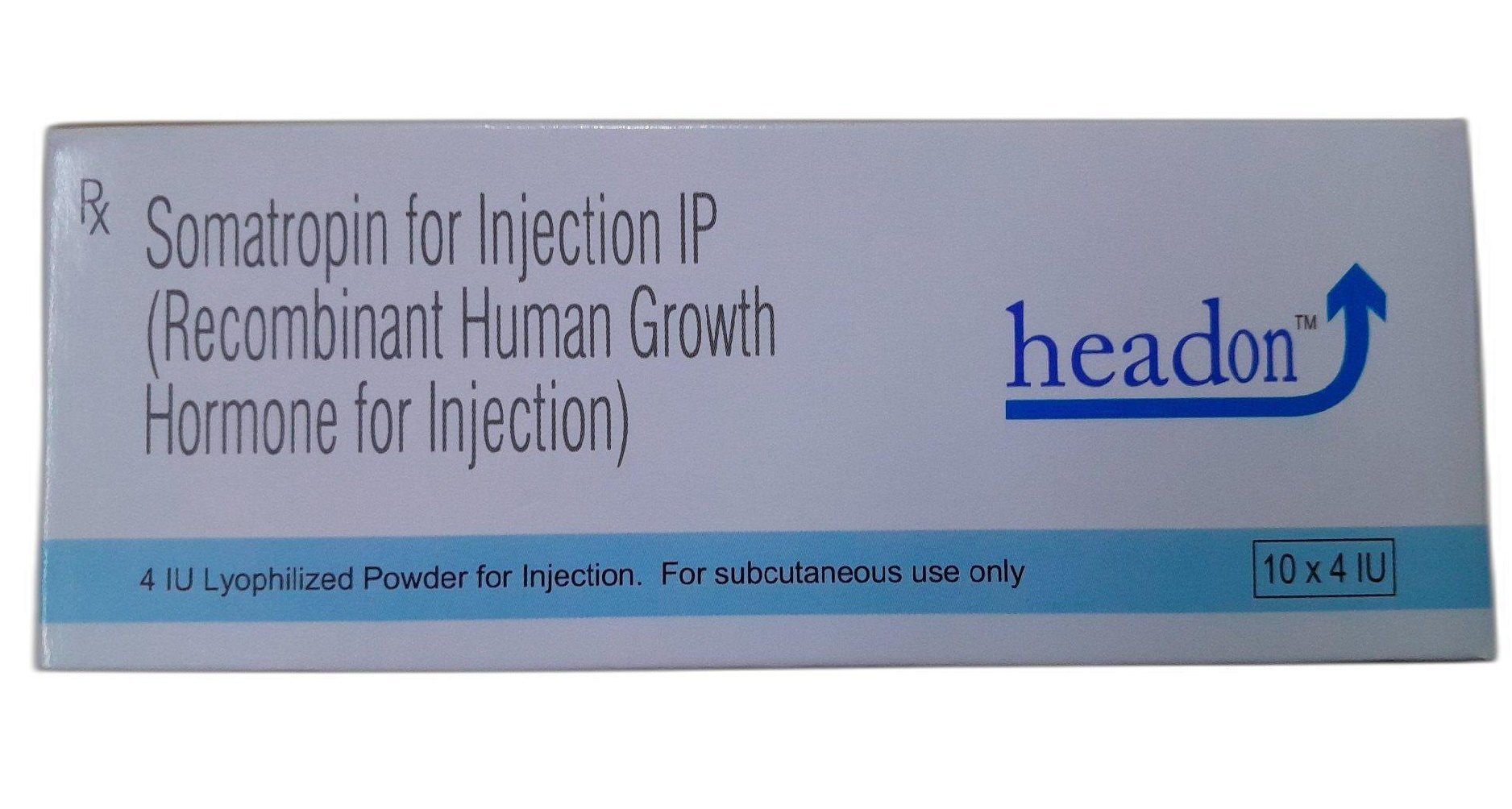
Headon 4IU Injection
₹1,093.75
Uses of Headon 4iu Injection
- Growth failure due to growth hormone deficiency
Introduction to Headon 4iu Injection
Headon 4IU Injection is a hormonal drug in the human growth hormone (hGH) agonist category, containing the active ingredient Somatropin. It treats growth failure in children due to deficiency of growth hormone, Turner’s syndrome, small for gestational age, and short stature of unknown cause. Apart from treating growth hormone deficiency, treatment with this medication also helps improve muscle mass, exercise capacity, bone mineral density and decreases fat mass.
This drug is contraindicated in severe obesity, acute critical illness, upper airway obstruction, sleep apnea, cancer, diabetes, impaired glucose tolerance, hypersensitivity, hypothyroidism, hypoadrenalism, scoliosis, and pancreatitis. Contact your doctor immediately if you experience breathlessness, rapid heartbeat, severe rash, swelling of arms or legs, or any other anaphylactic reactions. During treatment with Headon 4IU injection, your doctor may periodically monitor your blood count, blood sugar, thyroid, and other vital parameters to prevent serious complications.
Report to your doctor if you have had a history of diabetes, heart problems, hypothyroidism, pancreatitis, cancer, obesity, or acute illnesses. It is not known whether treatment with this drug alters fertility. Consult your doctor for more advice. Headon 4IU injection is not safe to be used during pregnancy. Report to your doctor if you are pregnant, suspecting, or planning for the pregnancy before starting the treatment. It is highly advised to use this injection with caution during breastfeeding.
Therapeutic Effects of Headon 4iu Injection
Headon 4IU injection is equivalent to the human pituitary hormone that helps in growth. It regulates the amount of insulin-like growth factor-I (IGF-I) and improves linear growth in patients with growth hormone deficiency.
Interaction of Headon 4iu Injection with other drugs
Inform your physician about any prescribed medications, over-the-counter medicines, nutritional or vitamin supplements, and herbal products you take or have taken before the treatment. Certain medications may interact with Headon 4IU injection and cause undesirable side effects.
More Information about Headon 4iu Injection
- Store Headon 4IU injection in a refrigerator (2°C-8°C).
- Do not freeze.
How to consume Headon 4iu
Headon 4IU will be given under the skin (subcutaneously). Your doctor, nurse, or other healthcare professional will show you how to inject the medicine. Do not try to administer the medicine if you are well-trained. Your physician will decide the injection dose based on your disease condition and severity.
Safety Advices for Headon 4iu
Pregnancy
Unsafe
Headon 4IU is not safe to be used during pregnancy. Report to your doctor if you are pregnant, suspecting, or planning for the pregnancy before starting the treatment.
Breast Feeding
Unsafe
Headon 4IU is used with caution in breastfeeding women. Consult with your physician for advice.
Lungs
Consult your doctor
It is unknown whether Headon 4IU is safe for patients with lung problems. Contact your doctor for more advice.
Liver
Consult your doctor
It is unknown whether Headon 4IU is safe for patients with liver problems. Contact your doctor for more advice.
Alcohol
Consult your doctor
It is unknown whether consuming alcohol while taking a Headon 4IU is safe. Please speak with your physician.
Driving
Safe
Headon 4IU does not affect your ability to drive and operate heavy machines.
Side Effects of Headon 4iu
Side Effects are unwanted symptoms caused by medicines. Although all medicines cause side effects, not everyone gets them.
Serious
- Visual changes
- Intracranial hypertension
Common
- Swelling
- Arthralgia
- Upper respiratory tract infection
- Pain
- Edema
- Paraesthesia
- Headache
- Stiffness
- Tiredness
- Muscle pain
- Back pain







Reviews
There are no reviews yet.